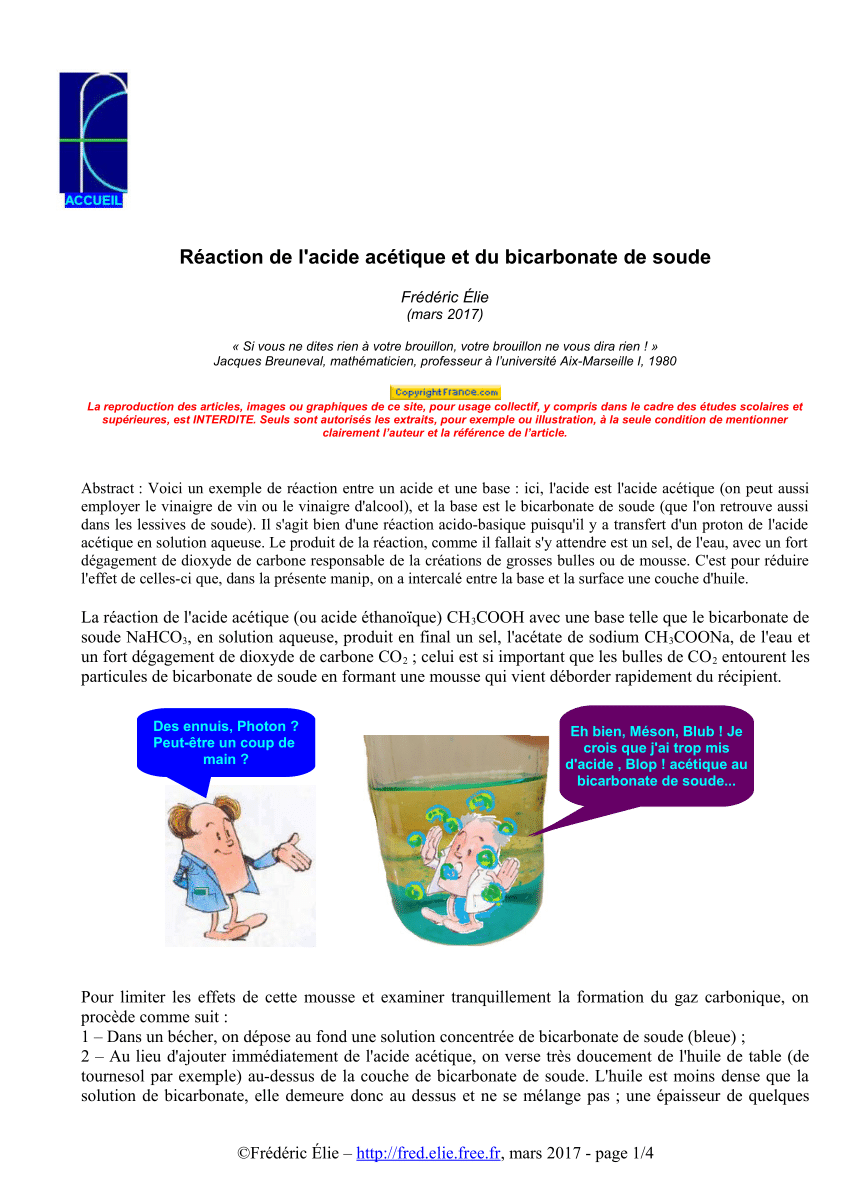
Nature Chose Phosphates and Chemists Should Too: How Emerging P(V) Methods Can Augment Existing Strategies | ACS Central Science

Nature Chose Phosphates and Chemists Should Too: How Emerging P(V) Methods Can Augment Existing Strategies | ACS Central Science

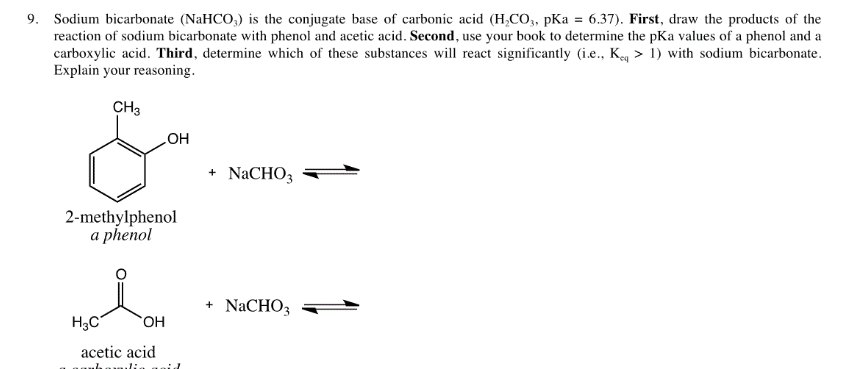
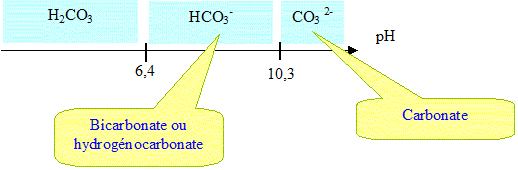
Water Mediated Wittig Reactions of Aldehydes in the Teaching Laboratory: Using Sodium Bicarbonate for the in Situ Formation of Stabilized Ylides | Journal of Chemical Education

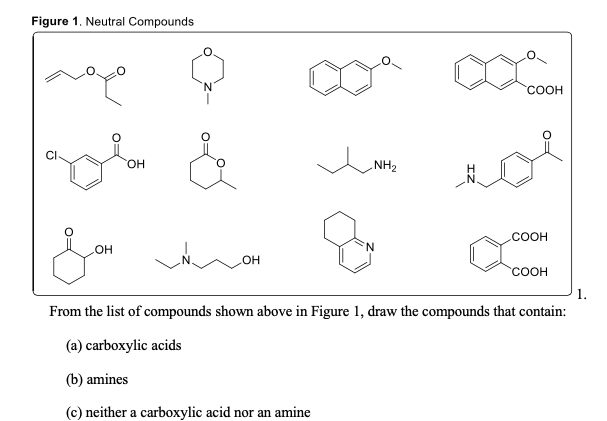
Schematic drawing of the important chemistry of sodium in the upper... | Download Scientific Diagram

EP0497065A1 - Industrial process for the preparation of phosphonic derivatives of vinblastine - Google Patents

Water Mediated Wittig Reactions of Aldehydes in the Teaching Laboratory: Using Sodium Bicarbonate for the in Situ Formation of Stabilized Ylides | Journal of Chemical Education